Properties of R744 – general system and design criteria
Carbon dioxide – CO2 – is a natural component of the air we breathe. The average concentration in the atmosphere is 400–420 ppm. Used as a refrigerant, carbon dioxide carries the ISO817/ASHRAE34 nomenclature R744.
Chemical properties:
R744 is highly soluble in water. At a temperature of 15°C and a pressure of 1 bar, 1 dm³ of water dissolves 1 dm³ of R744. When dissolved in water, it is called carbonic acid. As a gas, it is chemically and thermally stable enough for use as a refrigerant.
Physical properties:
R744 is an odourless and colourless gas. The CO2 molecule is not polar. R744 is non-toxic and non-flammable.
Molar mass | 44.01 kg/kmol | Unit |
Critical point | 73.77 bar +30.98°C | bar °C |
Triple point | 5,20 -56,60 | bar °C |
Sublimation point | 0,98 -78,90 | bar °C |
Saturation temperature | -10°C | 0°C | 20°C | Unit |
|---|---|---|---|---|
Saturation pressure | 26,49 | 34,85 | 57,29 | bar |
Liquid density | 982,93 | 927,43 | 773,39 | kg/m3 |
Saturated vapor density | 71,18 | 97,65 | 194,20 | kg/m3 |
Volumetric refrigerating capacity | 18409,45 | 22546,12 | 29518,04 | kg/m3 |
Isentropic exponent | 1,26 | 1,26 | 1,30 | |
Specific heat capacity, vapor cp | 1,51 | 1,86 | 4,56 | kJ/kg K |
Specific heat capacity, vapor cv | 0,81 | 0,87 | 1,07 | kJ/kg K |
Heat conductivity, boiling curve | 0,12 | 0,11 | 0,09 | W/m K |
Heat conductivity, condensation curve | 0,02 | 0,02 | 0,04 | W/m K |
Phase diagram and phase transition for R744:
solid -> liquid | Melting |
liquid -> solid | Freezing |
liquid -> gaseous | Evaporation |
gaseous -> liquid | Condensing |
solid -> gaseous | Sublimation |
gaseous -> solid | Resublimation |
Safety-relevant properties:
- According to EN378-1, R744 is a refrigerant in safety class A1, i.e. toxicity class A "non-toxic" and flammability class 1 "non-flammable".
- R744 has a suffocating effect in higher concentrations. Higher concentrations of R744 in the air we breathe reduce the absorption of atmospheric oxygen in the lungs.
- Due to its high density, R744 displaces atmospheric oxygen in enclosed spaces or near the ground.
- R744 is odourless and colourless, not directly perceptible in case of emission.
Workplace exposure limits (WELs): | 5000 ppm/volumetric |
Short-term exposure limit: | 10000 ppm/volumetric |
Immediate Danger to Life or Health (IDLH): | 50000 ppm/volumetric |
Thermodynamic properties:
- The specific volume of the liquid phase of R744 increases with rising temperature, more so than with other common refrigerants.
- In closed-off areas of a system, this property can lead to a safety-relevant increase in pressure as soon as there is no more space/free volume available for the expansion of the liquid.
- The following figure shows the pressure increase in an R744 refrigerant cylinder with increasing temperature for two filling ratios. At a temperature of 20°C, the saturation pressure is 57 bar (readable from the green boiling point curve). At 22.2°C, the cylinder is completely charged with a filling ratio of 0.75 kg/l. A further increase in temperature leads to an increase in pressure along the yellow function (isochoric change of state). R744 refrigerant cylinders are designed for a maximum pressure of 180 bar. This pressure is reached at a temperature of 50°C!
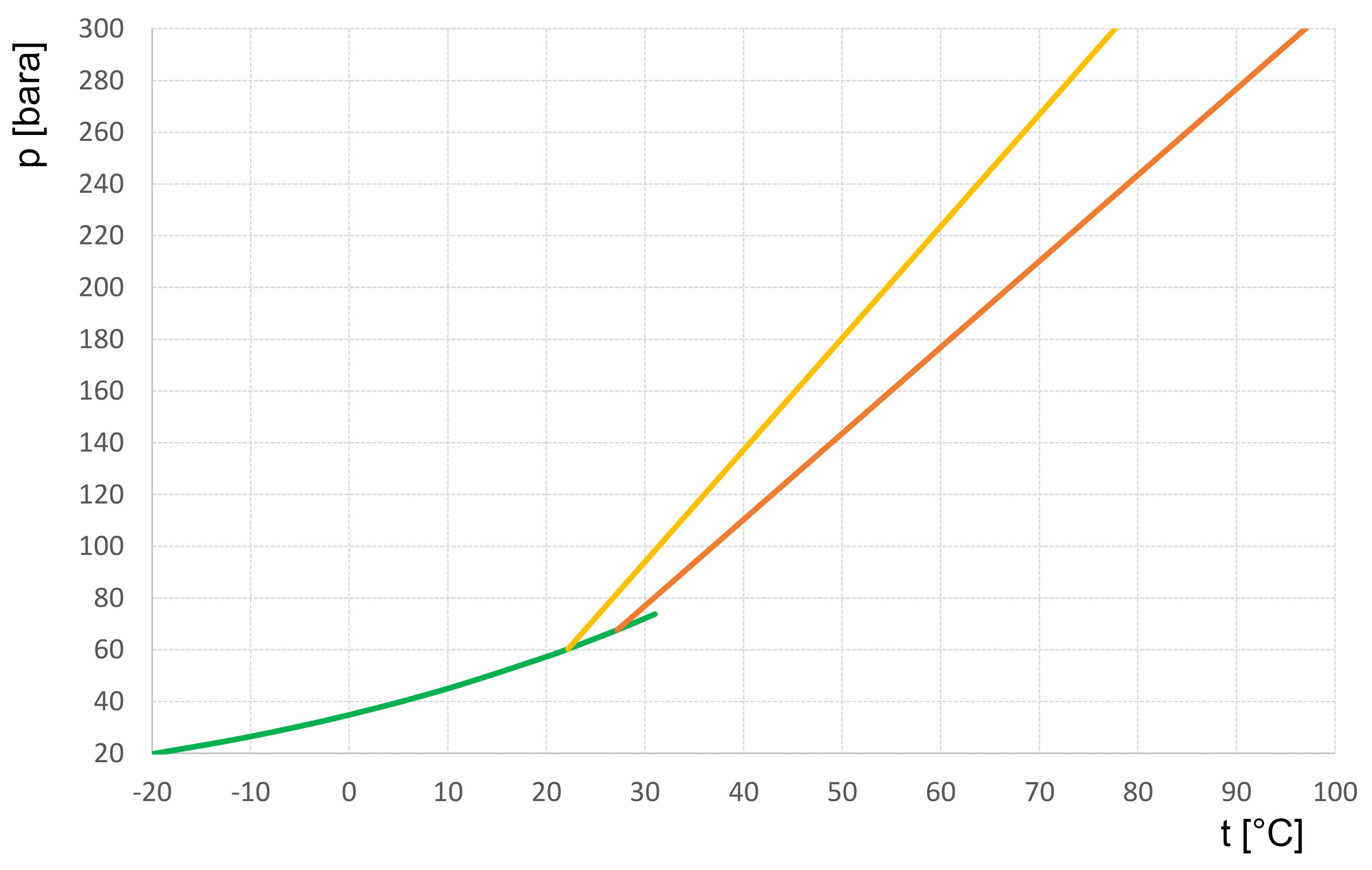
filling ratios
Green | Saturated liquid (boiling curve) |
Yellow | Filling ratio 0.75 kg/l: 100% at 22.2°C = 59.3 bar |
Red | Filling ratio 0.67 kg/l: 100% at 27°C = 65.5 bar |
as a function of temperature
Green | Specific volume of the liquid R744 | Green dashed | Saturation pressure of R744 |
Yellow | Specific volume of the liquid R717 | Blue | Specific volume of the liquid R134a |
Due to the low critical temperature of the refrigerant R744, heat dissipation occurs at high heat sink temperatures in the supercritical range, i.e. above the critical point. Heat absorption in the evaporators, on the other hand, continues to take place in the subcritical range. The fact that the process takes place both below and above the critical point means that the process is referred to as a transcritical process (see following figure).
Green | Subcritical process |
Red | Transcritical process |
2-3 | Gas cooling supercritical, only sensible heat change |
4-1 | Subcritical heat absorption in the evaporator, latent and sensible heat change |
Because pressure and temperature above the critical point are independent of each other (only sensible heat change), the efficiency, or Coefficient of Performance (COP), for a constant gas cooler outlet temperature is a function of pressure (see following figure).
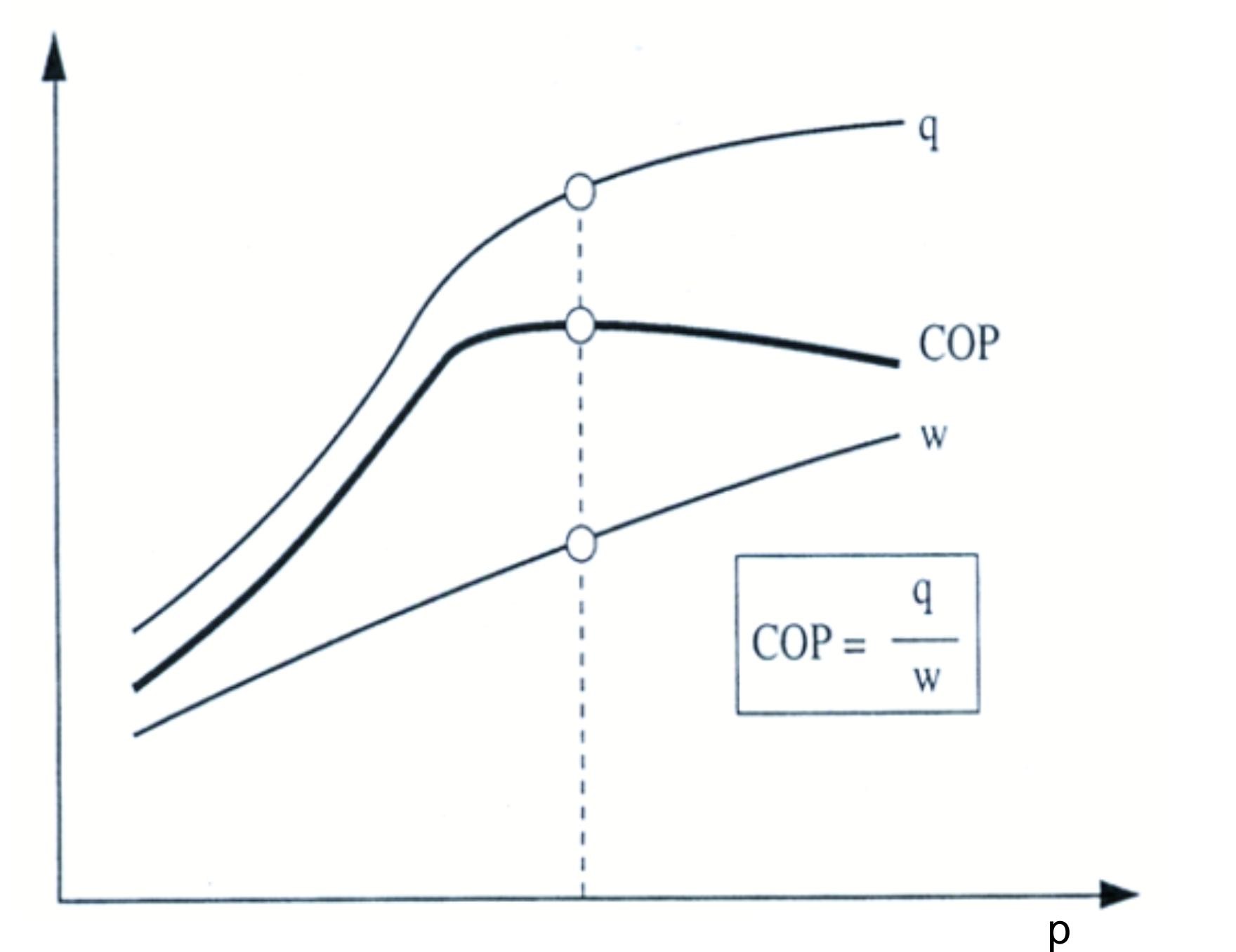
power consumption w, and coefficient of performance COP above the high pressure for
a constant gas cooler outlet temperature. Source: Sintef-NTNU